
As of May 11, 2020, there are 67,152 confirmed cases of COVID-19 in India. Since May 4, 24,619 new cases have been registered. Out of the confirmed cases so far, 20,917 patients have been cured/discharged and 2,206 have died. As the spread of COVID-19 has increased across the country, the central government has continued to announce several policy decisions to contain the spread, and support citizens and businesses who are being affected by the pandemic. In this blog post, we summarise some of the key measures taken by the central government in this regard between May 4 and May 11, 2020.
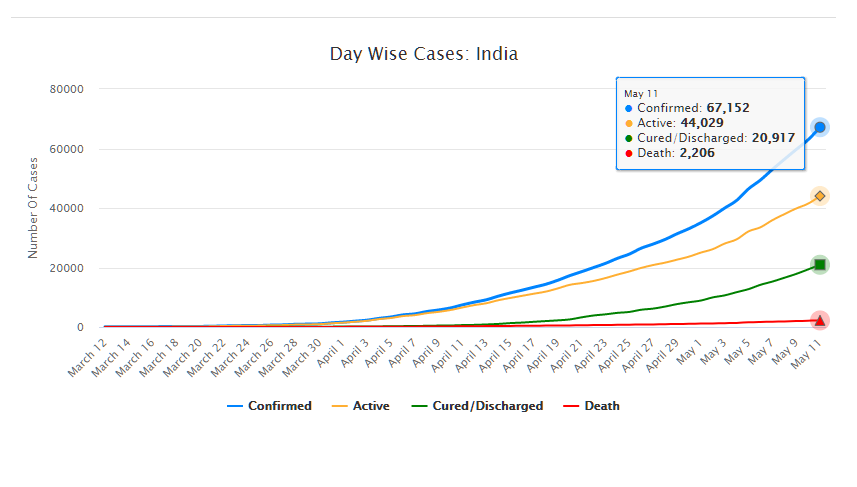
Source: Ministry of Health and Family Welfare; PRS.
Industry
Relaxation of labour laws in some states
The Gujarat, Himachal Pradesh, Rajasthan, Haryana, and Uttarakhand governments have passed notifications to increase maximum weekly work hours from 48 hours to 72 hours and daily work hours from 9 hours to 12 hours for certain factories. This was done to combat the shortage of labour caused by the lockdown. Further, some state governments stated that longer shifts would ensure a fewer number of workers in factories so as to allow for social distancing.
Madhya Pradesh has promulgated the Madhya Pradesh Labour Laws (Amendment) Ordinance, 2020. The Ordinance exempts establishments with less than 100 workers from adhering to the Madhya Pradesh Industrial Employment (Standing Orders) Act, 1961, which regulates the conditions of employment of workers. Further, it allows the state government to exempt any establishment or class of establishments from the Madhya Pradesh Shram Kalyan Nidhi Adhiniyam, 1982, which provides for the constitution of a welfare fund for labour.
The Uttar Pradesh government has published a draft Ordinance which exempts all factories and establishments engaged in manufacturing processes from all labour laws for a period of three years. Certain conditions will continue to apply with regard to payment of wages, safety, compensation and work hours, amongst others. However, labour laws providing for social security, industrial dispute resolution, trade unions, strikes, amongst others, will not apply under the Ordinance.
Financial aid
Central government signs an agreement with Asian Infrastructure Investment Bank for COVID-19 support
The central government and Asian Infrastructure Investment Bank (AIIB) signed a 500 million dollar agreement for the COVID-19 Emergency Response and Health Systems Preparedness Project. The project aims to help India respond to the COVID-19 pandemic and strengthen India’s public health system to manage future disease outbreaks. The project is being financed by the World Bank and AIIB in the amount of 1.5 billion dollars, of which one billion dollars is being provided by World Bank and 500 million dollars is being provided by AIIB. This financial support will be available to all states and union territories and will be used to address the needs of at-risk populations, medical personnel, and creating medical and testing facilities, amongst others. The project will be implemented by the National Health Mission, the National Center for Disease Control, and the Indian Council of Medical Research, under the Ministry of Health and Family Welfare.
Travel
Restarting of passenger travel by railways
Indian Railways plans to restart passenger trains from May 12 onwards. It will begin with 15 pairs of trains which will run from New Delhi station connecting Dibrugarh, Agartala, Howrah, Patna, Bilaspur, Ranchi, Bhubaneswar, Secunderabad, Bengaluru, Chennai, Thiruvananthapuram, Madgaon, Mumbai Central, Ahmedabad and Jammu Tawi. Booking for reservation in these trains will start at 4 pm on May 11. Thereafter, Indian Railways plans to start more services on new routes.
Return of Indians stranded abroad
The central government will facilitate the return of Indian nationals stranded abroad in a phased manner beginning on May 7. The travel will be arranged by aircraft and naval ships. The stranded Indians utilising the service will be required to pay for it. Medical screening of the passengers will be done before the flight. On reaching India, passengers will be required to download the Aarogya Setu app. Further, they will be quarantined by the concerned state government in either a hospital or a quarantine institution for 14 days on a payment basis. After quarantine, passengers will be tested for COVID-19 and further action will be taken based on the results.
For more information on the spread of COVID-19 and the central and state government response to the pandemic, please see here.
A recent news report stated that the Planning Commission has advocated putting in place a “proper regulatory mechanism” before permitting the use of genetic modification in Indian crops. A recent Standing Committee report on genetically modified (GM) crops found shortcomings in the regulatory framework for such crops. The current framework is regulated primarily by two bodies: the Genetic Engineering Appraisal Committee (GEAC) and the Review Committee on Genetic Manipulation (RCGM). Given the inadequacy of the regulatory framework, the Standing Committee recommended that all research and development activities on transgenic crops be carried out only in containment (in laboratories) and that ongoing field trials in all states be discontinued. The blog provides a brief background on GM crops, their regulation in India and the key recommendations of the Standing Committee. What is GM technology? GM crops are usually developed through the insertion or deletion of genes from plant cells. Bt technology is a type of genetic modification in crops. It was introduced in India with Bt cotton. The debate around GM crops has revolved around issues of economic efficacy, human health, consumer choice and farmers’ rights. Some advantages of Bt technology are that it increases crop yield, decreases the use of pesticides, and improves quality of crops. However, the technology has also been known to cause crop loss due to resistance developed by pests and destruction of local crop varieties, impacting biodiversity. Approval process for commercial release of GM crops
Committee’s recommendations for strengthening the regulatory process The Standing Committee report found several shortcomings in the regulatory framework, some of which are as follows:
Note that over the last few sessions of Parliament, the government has listed the Biotechnology Regulatory Authority Bill for introduction; however the Bill has not been introduced yet. The Bill sets up an independent authority for the regulation of GM crops. For a PRS summary of the report and access to the full report, see here and here.