
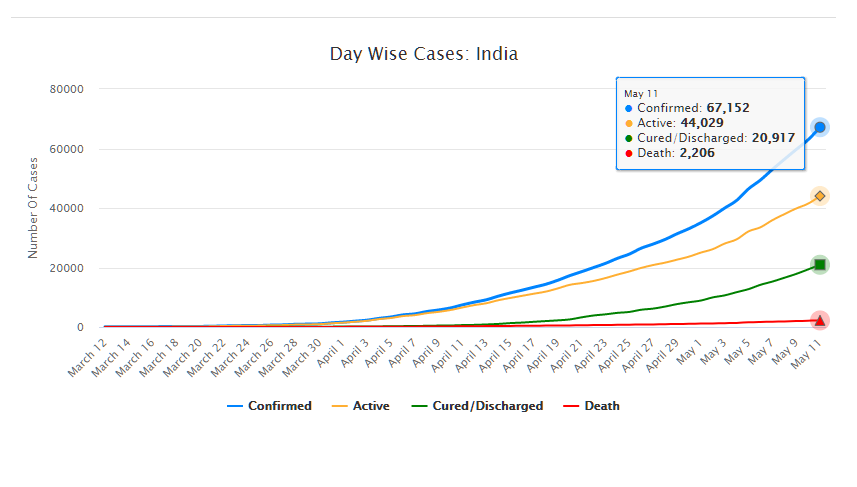
As of May 11, 2020, there are 67,152 confirmed cases of COVID-19 in India. Since May 4, 24,619 new cases have been registered. Out of the confirmed cases so far, 20,917 patients have been cured/discharged and 2,206 have died. As the spread of COVID-19 has increased across the country, the central government has continued to announce several policy decisions to contain the spread, and support citizens and businesses who are being affected by the pandemic. In this blog post, we summarise some of the key measures taken by the central government in this regard between May 4 and May 11, 2020.
Source: Ministry of Health and Family Welfare; PRS.
Industry
Relaxation of labour laws in some states
The Gujarat, Himachal Pradesh, Rajasthan, Haryana, and Uttarakhand governments have passed notifications to increase maximum weekly work hours from 48 hours to 72 hours and daily work hours from 9 hours to 12 hours for certain factories. This was done to combat the shortage of labour caused by the lockdown. Further, some state governments stated that longer shifts would ensure a fewer number of workers in factories so as to allow for social distancing.
Madhya Pradesh has promulgated the Madhya Pradesh Labour Laws (Amendment) Ordinance, 2020. The Ordinance exempts establishments with less than 100 workers from adhering to the Madhya Pradesh Industrial Employment (Standing Orders) Act, 1961, which regulates the conditions of employment of workers. Further, it allows the state government to exempt any establishment or class of establishments from the Madhya Pradesh Shram Kalyan Nidhi Adhiniyam, 1982, which provides for the constitution of a welfare fund for labour.
The Uttar Pradesh government has published a draft Ordinance which exempts all factories and establishments engaged in manufacturing processes from all labour laws for a period of three years. Certain conditions will continue to apply with regard to payment of wages, safety, compensation and work hours, amongst others. However, labour laws providing for social security, industrial dispute resolution, trade unions, strikes, amongst others, will not apply under the Ordinance.
Financial aid
Central government signs an agreement with Asian Infrastructure Investment Bank for COVID-19 support
The central government and Asian Infrastructure Investment Bank (AIIB) signed a 500 million dollar agreement for the COVID-19 Emergency Response and Health Systems Preparedness Project. The project aims to help India respond to the COVID-19 pandemic and strengthen India’s public health system to manage future disease outbreaks. The project is being financed by the World Bank and AIIB in the amount of 1.5 billion dollars, of which one billion dollars is being provided by World Bank and 500 million dollars is being provided by AIIB. This financial support will be available to all states and union territories and will be used to address the needs of at-risk populations, medical personnel, and creating medical and testing facilities, amongst others. The project will be implemented by the National Health Mission, the National Center for Disease Control, and the Indian Council of Medical Research, under the Ministry of Health and Family Welfare.
Travel
Restarting of passenger travel by railways
Indian Railways plans to restart passenger trains from May 12 onwards. It will begin with 15 pairs of trains which will run from New Delhi station connecting Dibrugarh, Agartala, Howrah, Patna, Bilaspur, Ranchi, Bhubaneswar, Secunderabad, Bengaluru, Chennai, Thiruvananthapuram, Madgaon, Mumbai Central, Ahmedabad and Jammu Tawi. Booking for reservation in these trains will start at 4 pm on May 11. Thereafter, Indian Railways plans to start more services on new routes.
Return of Indians stranded abroad
The central government will facilitate the return of Indian nationals stranded abroad in a phased manner beginning on May 7. The travel will be arranged by aircraft and naval ships. The stranded Indians utilising the service will be required to pay for it. Medical screening of the passengers will be done before the flight. On reaching India, passengers will be required to download the Aarogya Setu app. Further, they will be quarantined by the concerned state government in either a hospital or a quarantine institution for 14 days on a payment basis. After quarantine, passengers will be tested for COVID-19 and further action will be taken based on the results.
For more information on the spread of COVID-19 and the central and state government response to the pandemic, please see here.
Today, the National Medical Commission Bill, 2019 was passed by Lok Sabha. It seeks to regulate medical education and practice in India. In 2017, a similar Bill had been introduced in Lok Sabha. It was examined by the Standing Committee on Health and Family Welfare, which recommended several changes to the Bill. However, the 2017 Bill lapsed with the dissolution of the 16th Lok Sabha. In this post, we analyse the 2019 Bill.
How is medical education and practice regulated currently?
The Medical Council of India (MCI) is responsible for regulating medical education and practice. Over the years, there have been several issues with the functioning of the MCI with respect to its regulatory role, composition, allegations of corruption, and lack of accountability. For example, MCI is an elected body where its members are elected by medical practitioners themselves, i.e., the regulator is elected by the regulated. Experts have recommended nomination based constitution of the MCI instead of election, and separating the regulation of medical education and medical practice. They suggested that legislative changes should be brought in to overhaul the functioning of the MCI.
To meet this objective, the Bill repeals the Indian Medical Council Act, 1956 and dissolves the current MCI.
The 2019 Bill sets up the National Medical Commission (NMC) as an umbrella regulatory body with certain other bodies under it. The NMC will subsume the MCI and will regulate medical education and practice in India. Under the Bill, states will establish their respective State Medical Councils within three years. These Councils will have a role similar to the NMC, at the state level.
Functions of the NMC include: (i) laying down policies for regulating medical institutions and medical professionals, (ii) assessing the requirements of human resources and infrastructure in healthcare, (iii) ensuring compliance by the State Medical Councils with the regulations made under the Bill, and (iv) framing guidelines for determination of fee for up to 50% of the seats in the private medical institutions.
Who will be a part of the NMC?
The Bill replaces the MCI with the NMC, whose members will be nominated. The NMC will consist of 25 members, including: (i) Director Generals of the Directorate General of Health Services and the Indian Council of Medical Research, (ii) Director of any of the AIIMS, (iii) five members (part-time) to be elected by the registered medical practitioners, and (iv) six members appointed on rotational basis from amongst the nominees of the states in the Medical Advisory Council.
Of these 25 members, at least 15 (60%) are medical practitioners. The MCI has been noted to be non-diverse and consists mostly of doctors who look out for their own self-interest over public interest. In order to reduce the monopoly of doctors, it has been recommended by experts that the MCI should include diverse stakeholders such as public health experts, social scientists, and health economists. For example, in the United Kingdom, the General Medical Council which is responsible for regulating medical education and practice consists of 12 medical practitioners and 12 lay members (such as community health members, administrators from local government).
What are the regulatory bodies being set up under the NMC?
The Bill sets up four autonomous boards under the supervision of the NMC. Each board will consist of a President and four members (of which two members will be part-time), appointed by the central government (on the recommendation of a search committee). These bodies are:
How is the Bill changing the eligibility guidelines for doctors to practice medicine?
There will be a uniform National Eligibility-cum-Entrance Test for admission to under-graduate and post-graduate super-speciality medical education in all medical institutions regulated under the Bill. Further, the Bill introduces a common final year undergraduate examination called the National Exit Test for students graduating from medical institutions to obtain the license for practice. This test will also serve as the basis for admission into post-graduate courses at medical institutions under this Bill. Foreign medical practitioners may be permitted temporary registration to practice in India.
However, the Bill does not specify the validity period of this license to practice. In other countries such as the United Kingdom and Australia, a license to practice needs to be periodically renewed. For example, in the UK the license has to be renewed every five years, and in Australia it has to renewed annually.
How will the issues of medical misconduct be addressed?
The State Medical Council will receive complaints relating to professional or ethical misconduct against a registered medical practitioner. If the medical practitioner is aggrieved of a decision of the State Medical Council, he may appeal to the Ethics and Medical Registration Board. If the medical practitioner is aggrieved of the decision of the Board, he can approach the NMC to appeal against the decision. It is unclear why the NMC is an appellate authority with regard to matters related to professional or ethical misconduct of medical practitioners.
It may be argued that disputes related to ethics and misconduct in medical practice may require judicial expertise. For example, in the UK, the regulator for medical education and practice – the General Medical Council (GMC) receives complaints with regard to ethical misconduct and is required to do an initial documentary investigation in the matter and then forwards the complaint to a Tribunal. This Tribunal is a judicial body independent of the GMC. The adjudication decision and final disciplinary action is decided by the Tribunal.
How does the Bill regulate community health providers?
As of January 2018, the doctor to population ratio in India was 1:1655 compared to the World Health Organisation standard of 1:1000. To fill in the gaps of availability of medical professionals, the Bill provides for the NMC to grant limited license to certain mid-level practitioners called community health providers, connected with the modern medical profession to practice medicine. These mid-level medical practitioners may prescribe specified medicines in primary and preventive healthcare. However, in any other cases, these practitioners may only prescribe medicine under the supervision of a registered medical practitioner.
This is similar to other countries where medical professionals other than doctors are allowed to prescribe allopathic medicine. For example, Nurse Practitioners in the USA provide a full range of primary, acute, and specialty health care services, including ordering and performing diagnostic tests, and prescribing medications. For this purpose, Nurse Practitioners must complete a master's or doctoral degree program, advanced clinical training, and obtain a national certification.